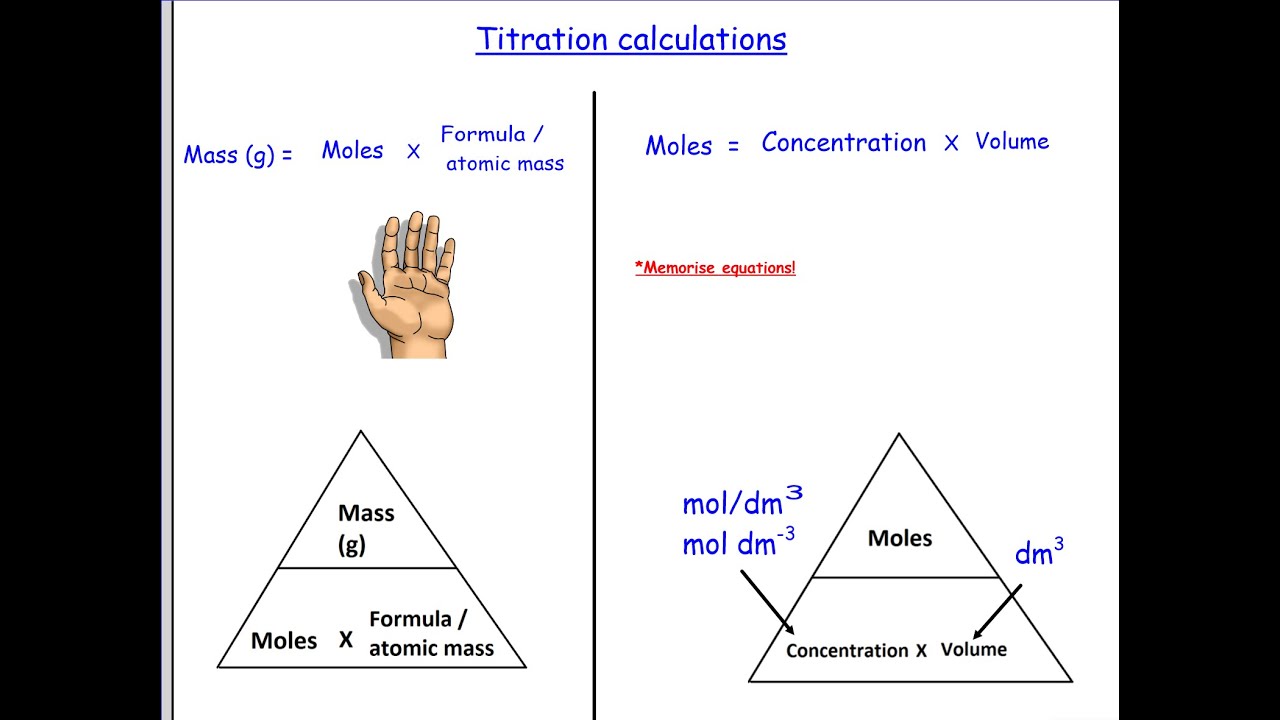
Titration Formula Ratio . A measured volume of the solution to be titrated, in this case,. Web the titration process can be observed in the video below. Determine the balanced chemical equation for the reaction taking place. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web here are the general steps for calculating titration: For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. Web use the titration formula. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
from www.youtube.com
If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web here are the general steps for calculating titration: Web use the titration formula. Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. Web the titration process can be observed in the video below. A measured volume of the solution to be titrated, in this case,. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Determine the balanced chemical equation for the reaction taking place. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the.
GCSE Chemistry Titration calculations worked examples YouTube
Titration Formula Ratio Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. Web here are the general steps for calculating titration: Web the titration process can be observed in the video below. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Determine the balanced chemical equation for the reaction taking place. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A measured volume of the solution to be titrated, in this case,. Web use the titration formula.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Formula Ratio A measured volume of the solution to be titrated, in this case,. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web the titration process can be observed in the video below. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the. Titration Formula Ratio.
From notariaurbina.cl
comoditate grup contrabandă titration calculations pdf chiriaş Radia pluti Titration Formula Ratio Web the titration process can be observed in the video below. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A measured volume of the solution to be titrated,. Titration Formula Ratio.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Formula Ratio For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web use the titration formula. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Web the titration process can be observed in the video below. Web in this equation the mole. Titration Formula Ratio.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Formula Ratio If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web use the titration formula. Web here are the general steps for calculating titration: Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A measured volume of the solution. Titration Formula Ratio.
From www.slideserve.com
PPT Calculating IV Titration in ml/hr PowerPoint Presentation, free Titration Formula Ratio Web here are the general steps for calculating titration: Determine the balanced chemical equation for the reaction taking place. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced. Titration Formula Ratio.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Formula Ratio Web use the titration formula. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A measured volume of the solution to be titrated, in this case,. Determine the balanced. Titration Formula Ratio.
From fity.club
Titration Formula Titration Formula Ratio Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web use the titration formula. A measured volume of the solution to be titrated, in this case,. Web in this. Titration Formula Ratio.
From www.sliderbase.com
Dilution Titration Formula Ratio Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Web use the titration formula. Determine the balanced chemical equation for the reaction taking place. Web here are the general steps for calculating titration: Web the titration process can be observed in the video below. If the titrant and. Titration Formula Ratio.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Formula Ratio If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. A measured volume of the solution to be titrated, in this case,. For a neutralization reaction with a 1:1. Titration Formula Ratio.
From fity.club
Titration Calculations Titration Formula Ratio For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web the titration process can be observed in the video below. A measured volume of the solution to be titrated, in this case,. Web use the titration formula. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m). Titration Formula Ratio.
From www.youtube.com
13 Titration Sample Calculation YouTube Titration Formula Ratio For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web the titration process can be observed in the video below. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A measured volume of the solution to be titrated, in this. Titration Formula Ratio.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration Formula Ratio For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web use the titration formula. Web here are the general steps for calculating titration: A measured volume of the solution to be titrated, in this case,. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the. Titration Formula Ratio.
From www.numerade.com
SOLVED 'What volume of 0.12M Ba(OH)2 is needed to neutralize 12.2 mL Titration Formula Ratio For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Determine the balanced chemical equation for the reaction taking place. Web use the titration formula. Web the titration process can be observed in the video below. If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the. Titration Formula Ratio.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Formula Ratio Web the titration process can be observed in the video below. Web here are the general steps for calculating titration: A measured volume of the solution to be titrated, in this case,. Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. Web use the titration formula. Web. Titration Formula Ratio.
From mungfali.com
Acid Base Titration Calculation Titration Formula Ratio Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. Web here are the general steps for calculating titration: A measured volume of the solution to be titrated, in this case,. Web use the titration formula. Web the titration process can be observed in the video below. If. Titration Formula Ratio.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Formula Ratio If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Determine the balanced chemical equation for the reaction taking place. Web in this equation the mole ratio of naoh (base) and hcl (acid) is. Titration Formula Ratio.
From mungfali.com
Acid Base Titration Calculation Titration Formula Ratio For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Web the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Web the titration process can be observed in the video below. If the titrant and analyte have a 1:1 mole ratio, the. Titration Formula Ratio.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Formula Ratio If the titrant and analyte have a 1:1 mole ratio, the formula is molarity (m) of the acid x. Web use the titration formula. A measured volume of the solution to be titrated, in this case,. Web in this equation the mole ratio of naoh (base) and hcl (acid) is 1:1 as determined by the balanced chemical equation. Web here. Titration Formula Ratio.